
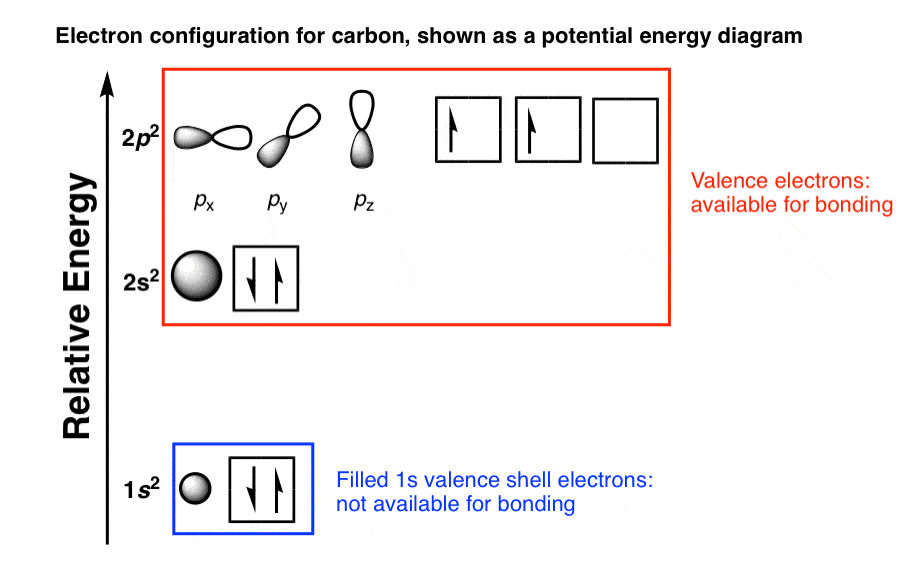
Typically, the sharing of electrons is observed to complete the valance shells by forming the multiple bonds with the other atoms. Carbon group the group 14 elements have four electrons in the outer shell. In neutral atoms, the number of electrons is equal to the number of the protons and atomic number can be easily determined by using the electron number. In the periodic table, elements are placed according to their atomic numbers that how many protons they have. Hund’s rule states that the pairing of electrons in the orbital only takes place when there is one electron in each subshell. The Pauli exclusion principle states that all four quantum numbers for any of the two electrons in an atom can never be the same. Another rule is defined by the Pauli and he defined a set of the unique quantum numbers for each of the electron. So, electrons fill the energy levels as defined by Aufbau’s principle. We can say that the valence electron of carbon is 4.Electrons fill the orbitals of the atom in such a way that the energy of the atom is at the minimum level. you can add then you can see that there are 4 valence electron for the carbon atom in second energy level. Write the order of higest energy, for electron configuration of carbon atom. Therefore, we can write the electron configuration for carbon will be,įinding the number of valence electron for the carbon, only you look highest energy in electron configuration. but in case of carbon atom only need 2 electrons. and remaining 2 electrons for carbon atom will go in the 2p orbital. You know that, 2nd orbital has capable to hold only 2 electrons. and next 2 electrons for carbon atom will goes in the 2nd orbitals. since, 1s orbitals can only hold 2 electrons. we can write electron configuration for carbon atom, first 2 electrons will go in the first orbital. Now, if you loot at the electron configuration of carbon atom. Now, i m going to draw electron configuration for carbon atom. this is the number of porton.if neutral then we have 6 electron. if you look at the periodic table, you can see that the atomic number of carbon is 6. First need to draw the electron configuration of carbon atom.
Carbon electron configuration number how to#
Here, i m going to discuss about how to identify valence electron for carbon atom. this is also easier techniques for calculating valence electron for an atoms. The other method is use to finding valence electron for any different atoms is, use the electron configuration. it mean, valence electron in outermost shell is 4. The inner shell contain 2 electron out of 6. Lets know, with electron digram for carbon atom.Ītomic number of carbon atom is 6. this is the easiest and better method to find out the valence electron for any different atoms. so, according to above information, surely, we can say that carbon atom have 4 valence electrons. The first easiest methods for finding the number of valence electrons for carbon atom or any different atoms, first look at the periodic table, if you properly look at the periodic table then you can see that, carbon in group 14. how many valence electrons does carbon have? First Method: Read More – What is the valency of sulphur? sulfur electron configuration.
How many valence electrons does carbon have – there are 4 valence electron in carbon atom.


 0 kommentar(er)
0 kommentar(er)
